Ethanol: production, recovery and uses
CONTENTS
- Introduction
- Microorganisms
- Media
- Fermentation
- Recovery
INTRODUCTION
- Ethanol (also called ethyl alcohol, grain alcohol) is a chemical compound.
- The chemical formula is C2H5OH.
- Ethanol is volatile, flammable, colorless, liquid with slight characteristics odor.
- After 1906, when the industrial alcohol act was passed, the production of industrial alcohol become commercially feasible.
MICROORGANISMS
- The choice of fermentation organism for industrial alcohol production depends to some extent, on the type of carbohydrate present in the medium.
- Bacteria: clostridium, Zymomonas mobilis.
- Yeast: cerevisiae, candida spp.
- Filamentous fungi: Fusarium, Mucor sp.
- Starch and sugar raw material: Specially selected strains of Saccharomyces cerevisiae is used.
Strain selection
- Must grow rapidly and tolerant to high concentration of sugar.
- Must produce large amount of alcohol.
- Relatively resistant to alcohol.
MEDIA
The principal media for the commercial production of industrial alcohol are:
- Blackstrap molasses or corn.
- Grains.
- Sulfite waste liquor.
- Whey potatoes.
- Wood waste.
- Sugarcane or sugar beet.
Fermentation
- Industrial alcohol production is carried out in very large fermenters (up to 125,000 gallons) and inoculum for these fermenters is added in the 3 to 10%.
- Temperature of fermenters is initially between 21° to 27°C but fermentation raises the temperature 28 to 30°C.
- Fermentation lasts approx. 2 to 3 days.
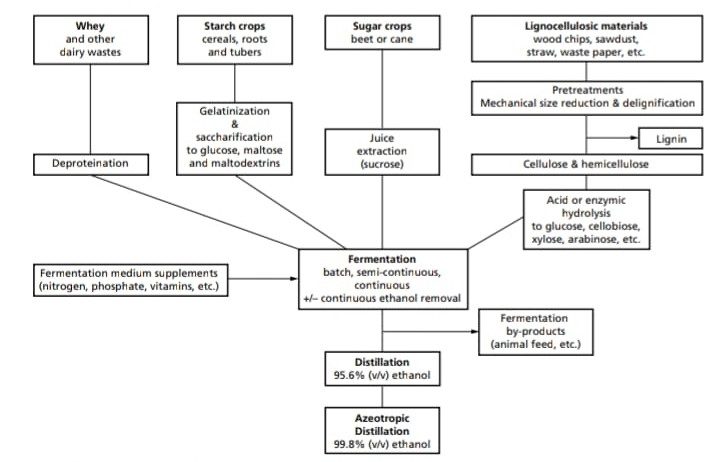
- Yeast cells are highly aerated to provide rapid cell multiplication.
- pH of the media ranges from 4.0 to 5.0 was the optimal for fermentations.
- The fermentation broth at completion of the fermentation contains in the range of 6 to 9% alcohol by volume and based on various reports, this alcohol yields reflects 90 to 98% theoretical conversion of substrate sugar to alcohol.
RECOVERY
Ethanol recovery is based on distillation:
-
- The broth is distilled in a beer column to harvest 85% v/v ethanol.
- The another step of rectification gives 96.5% ethanol, which is then, Dehydrated to 99.4% by using benzene or cyclohexane if the ethanol is to be used as a fuel blend.
- To obtain 100% it requires to form an azeotrophic mixture containing 5% water, thus 5% water is removed from mixture of ethanol water and benzene after distillation azeotrophic.
- In this process, benzene, water, ethanol and then ethanol benzene, azeotrophic mixtures to be removed, so that absolute alcohol is obtained.
USES
- Antiseptics: ethanol has a bactericidal and antifungal activity so, it commonly used in antibacterial hand sanitizer and as a antiseptics and disinfectants.
- Antidote: It is also administered as an antidote to different poisoning such as methanol, isopropyl alcohol and ethylene glycol.
- Fuel: it is largely used as an engine fuel and fuel additive.
- Rocket fuel: it was used as fuel in liquid propelled vehicle.
- Solvent: it is considered universal solvent because it allows dissolving of both polar, hydrophilic and non polar, hydrophobic.
- Ethanol is commonly and largely used as beverages for drinking purpose as well as in paints, tincture, etc.
- Also used as medical solvent.
Also Read:
- Beer brewing process
- Microbial Fuel Cells
- Electrophoresis: Overview, Principles and Types
- Citric acid: Introduction, Fermentation, Recovery and Uses
- Cider: Production, Extraction, Fermentation and Maturation
- Malaria: Causative Agent, Symptoms, Treatment and Prevention
- Gonorrhea: Causative Agent, Symptoms, Treatment and Prevention
- Reverse Transcription Polymerase Chain Reaction (RT-PCR)
- Comparison Between the Domains Bacteria, Archaea, and Eukarya
- Fundamental Principle of Clinical Specimen Collection
- Microbial Identification and Strain Typing Using Molecular Techniques
