Fundamental Principle of Clinical Specimen Collection
Content
- Introduction
- Fundamental Principles of Specimen Collection
- Collection Procedures
- Patient-Collected Specimens
- Labeling and Requisitions of sample
- Safety
Introduction of Clinical Specimen Collection
- The laboratory can make accurate and useful determinations only if a specimen has been collected properly.
- The specimens to be analyzed are likely to contain living organisms; the goal of the specimen collector must be to maintain the viability of these organisms with minimal contamination.
Fundamental Principles of Specimen Collection
The following basic principles of specimen collection are fundamental to ensuring appropriate specimen management:
- If possible, collect the specimen in the acute phase of the infection and before antibiotics are administered.
- Select the correct anatomic site for collection of the specimen.
- Collect the specimen using the proper technique and supplies with minimal contamination from normal biota (normal flora).
- Collect the appropriate quantity of specimen.
- Package the specimen in a container or transport medium designed to maintain the viability of the organisms and avoid hazards that result from leakage.
- Label the specimen accurately with the specific anatomic site and the patient information patient’s name and a unique identification number.
- Transport the specimen to the laboratory promptly or make provisions to store the specimen in an environment that will not degrade the suspected organism(s).
- Notify the laboratory in advance if unusual pathogens or agents of bioterrorism are suspected.
Collection of Clinical Specimen Procedures
- Specimens for microbiology cultures should be collected in sterile containers except for stool specimens, which can be collected in clean, leak proof containers.
- Generally, swabs are not recommended for collection because they do not provide sufficient quantity, are easily contaminated, and can become dried out leading to a loss of organisms.
- Swabs are appropriate for specimens from the upper respiratory tract, external ear, eye, and genital tract.
- The tips of swabs may contain cotton, Dacron, or calcium alginate. Cotton-tipped swabs tend to have excessive fatty acids, which may be toxic to certain bacteria.
- Dacron or polyester swabs have a wide range of uses.
- Swab collection systems are available that provide transport media and protect the specimen from drying.
- Lesions, wounds, and abscesses present many problems to the microbiology laboratory.
- The term wound is not an appropriate specimen label, and the exact anatomic site must be provided.
- The specimen is collected from the advancing margin of the lesion and should be collected by needle aspiration rather than by swab.
- Before the specimen is collected, the area should be cleansed to eliminate as much of the commensal flora as possible.
- Aspirated material should be placed into a sterile tube or transport vial and not “squirted” onto a swab.
Patient-Collected Specimens
- In certain situations, patients are asked to collect the specimen themselves.
- Medical personnel should provide patients with thorough instructions on how to collect the sample.
- It should not be assumed that the patient knows how to collect a particular type of specimen.
- Attaching printed instructions in multiple languages to a collection device does not ensure that patients will read them or understand them.
- The most effective method is to provide verbal and written instructions.
- It may be necessary to read the instructions to the patient.
- The instructions should be written using simple language and pictures to help the patient understand the procedure as it is verbally explained.
The specimens commonly obtained by the patient are urine, sputum, and stool:
Urine collection
- Instructions for urine collection must include an explanation of the clean-catch midstream urine specimen.
- A first morning specimen is preferred because it provides a more concentrated sample.
- The patient collects this specimen following cleansing of the external genitalia to reduce the presence of indigenous flora.
- Patients are asked to void without collecting the first portion of the urine flow and instead to collect the middle portion.

- The first portion of the urine flow washes contaminants from the urethra, and the midstream portion is more representative of the urine in the bladder.
- Personnel who collect catheterized specimens should also use this technique to eliminate organisms carried up the urethra during catheterization.
Sputum collection
- Sputum specimens are often collected for the diagnosis of bacterial pneumonia.
- Lower respiratory tract specimens are among the most difficult specimens to collect adequately because they are contaminated with oropharyngeal flora.
- For this reason, they are one of the least clinically relevant specimens received for culture.
- Other specimens, such as blood or a bronchoalveolar lavage (BAL), may be more accurate in detecting the etiologic agent (i.e., the microorganism causing the disease).
- Collection of a quality sputum sample requires thorough patient education and medical personnel oversight of the process.
- The first early morning specimen is preferred.
- The patient needs to understand the difference between sputum, saliva, and nasal secretions.
- The patient should rinse the mouth with water and expectorate with the aid of a deep cough directly into a sterile container (expectorated sputum).
- Patients with dentures should remove the dentures first.
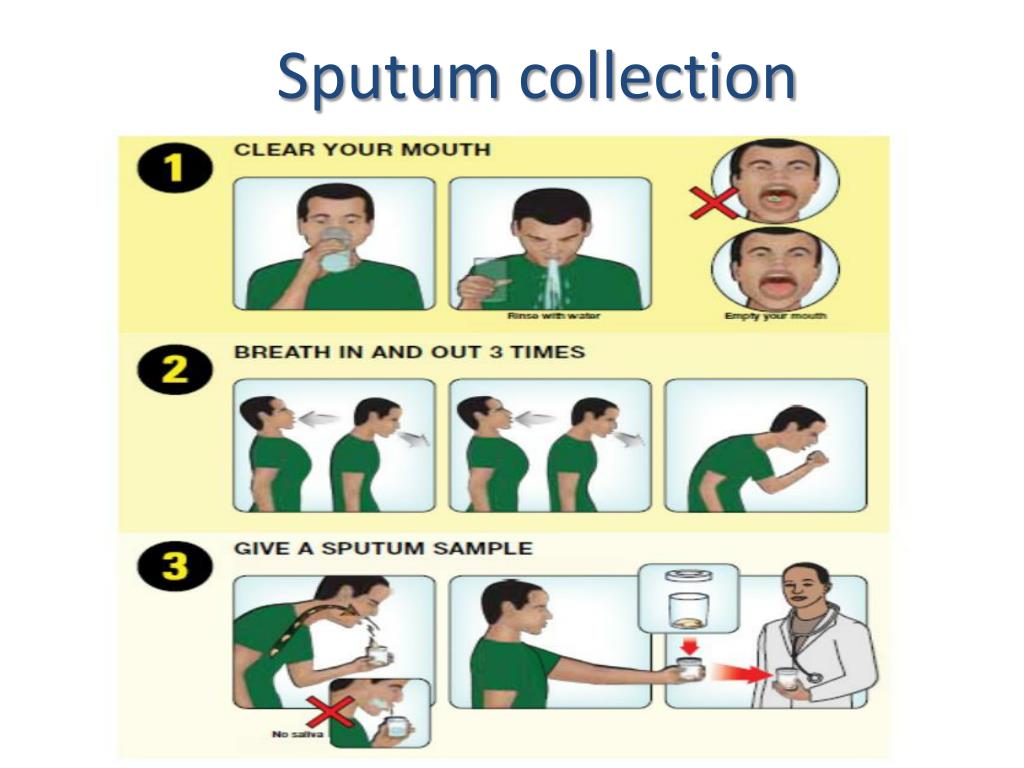
- A single specimen should be adequate for detection of bacterial lower respiratory tract infection.
- If fungal or mycobacterial infections are suspected, three separate early morning specimens are appropriate.
- Respiratory therapy technicians may assist patients who are unable to expectorate a respiratory specimen.
- These specimens may be collected through aerosol induction, in which the patient breathes aerosolized droplets of a solution that stimulates cough reflex (induced sputum).
- When sputum specimens are submitted to microbiology, the laboratory should be informed of whether the specimen is expectorated or induced.
Stool collection
- The specimen of choice for the detection of gastrointestinal pathogens is stool.
- A rectal swab can be submitted for bacterial culture as long as fecal material is visible on the swab.
- A single specimen that has yielded a negative result is not usually sufficient to exclude bacteria or parasites.
- If a bacterial infection is suspected, three specimens should be collected—one a day for 3 days.
- If parasites are suspected, three specimens collected within 10 days should be sufficient for microscopic detection of ova and parasites.
- Some laboratories offer an initial parasite screening for Giardia lamblia and Cryptosporidium spp.
- Newer methods detect parasite antigens, and one sample is usually sufficient.
- If the screen is negative, the physician can decide whether to perform complete parasite studies.
- Patients should be instructed to excrete directly into the collection device.

- Specimens should never be taken from the toilet and should not be contaminated with urine.
- Commercial systems are available with preservatives for bacteria and parasites.
- The appropriate ratio of stool to preservative is 1 : 3, and the patient must understand that if this ratio is not met, the test will be invalid.
- In addition, the patient needs to be told that the specimen must be thoroughly mixed with the preservative.
- Specimens for parasite microscopic studies should be collected before any barium studies are done; if this is not feasible, the patient must delay specimen collection until the barium is cleared (4 to 5 days).
- Barium appears as a white chalky substance in the specimen and masks the appearance of parasites under the microscope.
Labeling and Requisitions of clinical sample
- It is important that correct patient identification to be put on the specimen and the requisition.
- The specimen label must contain sufficient information for the specimen and requisition to be matched up when received in the laboratory.
- The laboratory loses valuable time when specimens are unlabeled or mislabeled.
Proper identification of each specimen includes a label attached to the container with the following information:
-
- Name
- Identification number
- Room number
- Physician
- Culture site
- Date of collection
- Time of collection
- To perform quality laboratory analysis, the laboratory needs specific information regarding the patient and the specimen.
- All that the laboratory knows about the patient is learned from the requisition form. The less information that is provided, the more difficult it is for the laboratory to provide good patient care.
- Incomplete information on the requisition is often a weak link in the specimen management process.
The requisition form should provide the following information:
-
- Patient’s name
- Patient’s age (or date of birth) and gender
- Patient’s room number or location
- Physician’s name and address
- Specific anatomic site
- Date and time of specimen collection
- Clinical diagnosis or relevant patient history
- Antimicrobial agents (if the patient is receiving)
- Name of individual transcribing orders
- Complete and thorough requisitions can often lead the microbiology technologist to suspect certain pathogens based on the diagnosis or patient history.
- This knowledge can allow use of specific media or making certain adjustments to the incubation to maximize recovery of the pathogen.
- Computer-based ordering is performed at many institutions. Ideally, the microbiology technologist should design the testordering process; this will enable the laboratory to elicit the necessary information.
- These systems should be designed to provide key fields that must be completed to submit the request transaction.
- The microbiologist should recognize that the individual ordering the test does not have a complete knowledge of what are and are not appropriate tests for each specimen.
- If the test requested is not recommended, it is the responsibility of the laboratory to communicate with the physician to determine exactly what needs to be done.
Safety during clinical specimen collection
- It is imperative that specimens collected for microbiology not pose a safety hazard to the individuals who handle them.
- Leaking containers and specimens with needles attached present the greatest hazards.
- All specimens must be transported in leak proof secondary containers.
- Because the specimen should be kept separate from any paperwork, plastic bags with permanent seals and separate pouches on the outside for requisitions are recommended.
- Transporting personnel should refuse to transport specimens without the protection of a secondary container. Refusing to accept syringes with needles attached is also appropriate.
- A needle must be replaced with a tight-fitting rubber stopper or a stopcock to put resistance on the plunger.
- The aspirated material could also be transferred to another sterile container with a tight lid or to an anaerobic transport system.
- Laboratory personnel must also adhere to strict safety guidelines as they begin to work with the patient’s specimen.
- All individuals handling patient specimens must wear protective clothing, and specimens should be opened only in a biological safety cabinet.
Reference and Sources
- https://www.scribd.com/presentation/327892603/Specimen-Collection-and-Processing
- https://www.medicinebau.com/uploads/7/9/0/4/79048958/specimens-collection.pdf
- https://www.questdiagnostics.com/home/physicians/testing-services/specialists/hospitals-lab-staff/specimenhandling/general/
- https://www.healthcare.uiowa.edu/path_handbook/Appendix/Micro/micro_spec_collection.html
- http://www.medicinebau.com/uploads/7/9/0/4/79048958/samples_collection.pdf
- https://quizlet.com/70277499/mls-321-practice-quiz-chpt-6-9-flash-cards/
- https://www.medicinebau.com/uploads/7/9/0/4/79048958/micro_sheet_12.pdf
- https://quizlet.com/88368578/specimen-collection-and-processing-flash-cards/
- https://quizlet.com/50751006/microbiology-module-2-flash-cards/
- https://quizlet.com/187871227/microbiology-exam-2-ch-67-flash-cards/
- https://www.researchgate.net/publication/11185160_Laboratory_Diagnosis_of_Lower_Respiratory_Tract_Infections_Controversy_and_Conundrums
- https://quizlet.com/38184661/microbiology-review-ascp-flash-cards/
- https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html
Also Read:
- Microscopy: Overview, Principles and Its Types
- Mesothelioma Lung Cancer
- AIDS: Acquired Immune Deficiency Syndrome
- Exposure and Transmission of Infectious Disease
- Nosocomial Infection: Introduction, Source, Control and prevention
- Reverse Transcription Polymerase Chain Reaction (RT-PCR)
- Syphilis: Agent, Epidemiology, Symptoms, Treatment and Prevention
- Gonorrhea: Causative Agent, Symptoms, Treatment and Prevention
- Chlamydia: Introduction, Epidemiology, Transmission, Treatment and Prevention
- Microbiology Disciplines: Bacteria, Viruses, Fungi, Archaea and Protists
