Production of Ethanol
Most regions of the world have traditionally produced alcoholic beverages from locally available substrates. Similar alcoholic fermentations are now used in some countries to produce fuel grade or chemical feedstock ethanol. The annual world production of ethanol is over 30 billion litres, approximately 70% of which is produced by fermentation, the remaining being mostly manufactured by the catalytic hydration of ethylene. Almost 12% of the fermentation ethanol is beverage alcohol, 20% is for various industrial uses and the remaining 68% is fuel ethanol.
Ethanol is an attractive fuel because it may be used alone or mixed with other liquid fuels, e.g. ‘gasohol’, a blend of 10–22% (v/v) ethanol with gasoline. In the 1970s, Brazil and a few other countries undertook the full-scale production of ethanol from indigenous renewable biomass resources to offset the growing costs of oil imports. The ethanol was produced by fermentation of sucrose, derived from sugar cane, using Saccharomyces cerevisiae.
Brazil is now responsible for over 46% of annual world production of ethanol, some 14.5 billion litres of ethanol. However, this is not sufficient to keep up with the growing demand for fuel. Failure to develop their production processes has resulted in the need to import ethanol from the USA and other producing countries.
Apart from sucrose, other conventional fermentation substrates for ethanol fermentations include simple sugars derived from plants and dairy wastes. These require relatively little processing. However, use of root and tuber starch (cassava, potato, etc.) or grain starch (maize, wheat, rice, etc.) demands energy-consuming processing operations to achieve hydrolysis. Even greater processing is necessary prior to the utilization of lignocellulosic plant materials.

Bioconversion of maize starch
In North America, wet or dry milling processes have been developed for maize processing to separate corn oil from the starch. This also generates byproducts that can be used for animal feed. Extracted starch is subjected to gelatinization and saccharification, and the resultant sugars can then undergo alcoholic fermentation. The technology initially used was largely based on that previously developed for alcoholic beverage production, but now the processes are much more efficient. Enzymatic saccharification is used for converting starch into fermentable sugars employing various thermostable amylases, including glucoamylases.
Fermentation of the resulting sugars, mostly glucose, is carried out by selected strains of S. cerevisiae at 32–38°C and pH 4.5–5.0. Fermentations may be batch or continuous processes, often with some form of cell recycle, which reduces both the fermentation time and the amount of substrate ‘wasted’ in conversion to unwanted biomass. Operation under vacuum, facilitating continuous removal of ethanol to reduce ethanol inhibition, and even cell immobilization have been trialled.
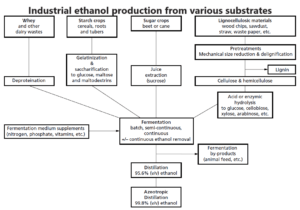
These alcoholic fermentations generate a ‘beer’ containing approximately 10% (v/v) ethanol from which the yeast is usually separated prior to distillation. Recovered ethanol may then be dehydrated Distillation). The cost of this ethanol recovery is often up to 50% of the total process expenditure. Process byproducts include methanol, glycerol and higher alcohols, such as amyl, butyl and propyl alcohols.
Possible alternate approaches to ethanol recovery include the use of continuous extractive fermentation processes using non-volatile, non-toxic solvents, such as oleyl alcohol, which have a high affinity for ethanol. This stategy is useful in overcoming end-product inhibition The solvents employed are continuously introduced into the fermenter and rise through the medium to form a layer that is continuously removed. Passage through a centrifuge results in the separation of ethanolladen solvent from media and cells, which are returned to the fermenter. The ethanol may be recovered by flash vaporization and the non-volatile solvent is reused.
Although S. cerevisiae is still extensively used for the alcoholic fermentation of simple sugar substrates, there are other organisms with commercial potential. These include species of the bacterial genus Zymomonas, such as Z. mobilis, which are Gram negative facultative anaerobes that normally ferment only glucose, fructose or sucrose. They afford greater ethanol yields than does S. cerevisiae, but are not as ethanol tolerant.
In future, alternate routes are likely to involve genetically engineered organisms that have the ability to utilize a wider range of carbon sources and have better fermentation properties. For example, Escherichia coli, which normally produces only relatively small amounts of ethanol, has been transformed with a plasmid that encodes the alcohol dehydrogenase and pyruvate decarboxylase from Z. mobilis. Such transformants produce ethanol under both aerobic and anaerobic conditions.
Ethanol-producing microorganisms
| Bacteria | |
| Clostridium thermohydrosulfuricum | Extreme thermophile |
| Clostridium thermocellum | Thermophilic, hydrolyses cellulose |
| Thermoanaerobacter ethanolicus | Ferments xylose and starch* |
| Zymomonas mobilis | The wild-type ferments only glucose, fructose and sucrose, but with high productivity |
| Yeasts | |
| Candida pseudotropicalis, C. tropicalis | Ferments xylose* |
| Candida species | Ferment xylose and cellobiose* |
| Kluyveromyces lactis† | Ferments lactose in dairy wastes* |
| Kluyveromyces marxianus | Hydrolyses inulin (polyfructosan) |
| Pachysolen tannophilus | Ferments xylose* |
| Pichia stipitis | Ferments xylose* |
| Saccharomyces cerevisiae | Most strains ferment only glucose, sucrose, fructose, maltose and maltotriose |
| S. cerevisiae var. distaticus | Hydrolyses starch |
| Schwanniomyces alluvius | Hydrolyses starch |
| Filamentous fungi | |
| Fusarium species | Ferment xylose* |
| Monilia species | Hydrolyse cellulose and xylan |
| Mucor species | Ferment xylose and arabinose* |
*In addition to common hexose monosaccharides and disaccharides. †Does not exhibit the Crabtree effect.
Bioconversion of lignocellulosic materials
Unlike energy crops (cereals, sugar cane and beet, etc.), lignocellulosic plant wastes (sawdust, wood chips, straw, bagasse, waste paper, etc.) have no direct food use. They are renewable resources yet to be fully exploited Billions of tonnes of these cellulosic materials
currently go to waste each year, which could be converted into chemical energy or other useful fermentation products.
Lignocellulose is composed of the following polymers:
- Lignin (10–35%, w/w), a polymer of three phenolic alcohols (p-coumaryl, sinapyl and coniferyl alcohols) that encrusts the cellulose. This material cannot be degraded by microorganisms under anaerobic conditions, but may be used as sources of vanillin, catechol, dimethylsulphide (DMS) and dimethyl sulphoxide (DMSO) via chemical processes.
- Cellulose (15–55%, w/w), a linear homopolymer of β-1,4-linked glucose units. Once hydrolysed, the resultant glucose is readily fermented by many microorganisms, but few can directly utilize the native polymer.
- Hemicellulose (25–85%, w/w), a class of heteropolymers containing various hexoses (D-glucose, D-galactose and D-mannose) and pentoses (L-arabinose and D-xylose). Xylose is the second most abundant sugar in nature after D-glucose and may constitute up to 25% of the dry weight of some woody trees, but only a few microorganisms ferment pentoses to ethanol. Importantly, ethanol production from lignocellulose is economically viable only if both pentoses and hexoses are fermented.
Few microorganisms can utilize lignocellulose directly and those that do, such as some species of Clostridium, produce little or no ethanol. Therefore, direct microbial fermentation of cellulosics to ethanol is a distant opportunity. An approach more likely to prove
successful in the shorter term involves several steps. First, pretreatment of the lignocellulosic material is necessary prior to the saccharification of hemicellulose and cellulose components. Sugars resulting from chemical and/or enzymic hydrolysis may then be fermented to produce ethanol, which can be separated from the aqueous phase by distillation.
Pretreatment and saccharification must be conducted in a way that maximizes subsequent bioconversion yields and minimizes the formation of potentially inhibitory compounds, especially furfurals and soluble phenolics. Most lignocellulosic materials require a pretreatment to render the cellulose and hemicellulose more amenable to acid or enzyme hydrolysis. Pretreatment requirements vary with the feedstock and are often substantially less for processed materials such as paper and card. Methods employed include mechanical size reduction by milling, chemical pulping, acid hydrolysis, alkali treatment, autohydrolysis, solvent extraction, steaming and steam explosion (explosive decompression following high-pressure steam treatment at 4000 kPa for 5–10 min).
Various combinations of pretreatment processes may be used depending upon the source of lignocellulosic materials. Some achieve partial saccharification, but further treatment with acid or enzymes is usually necessary. Acid hydrolysis is generally carried out with dilute acid (e.g. 0.5–5% (v/v) sulphuric acid) under pressure to achieve elevated temperatures of 100–240°C. This treatment is relatively inexpensive, but also generates large quantities of degradation byproducts and undesirable inhibitory compounds.
Strong acid hydrolysis often uses concentrated hydrochloric acid at ambient temperature, which gives the highest sugar yield of any acid hydrolysis process. However, such operations are highly corrosive and almost complete acid recovery is essential to make the process economically viable. Acid hydrolysis of mixtures of cellulose and hemicellulose is difficult to control. Hemicellulose is more readily hydrolysed than cellulose and generates sugars early in the process. These sugars may undergo further breakdown to inhibitory compounds, e.g. furfurals. Consequently, conditioning of hydrolysates may be necessary in order to remove these compounds, prior to fermentation.
The sugars generated by hydrolysis are primarily glucose, cellobiose (a disaccharide composed of β-1,4-linked glucose units) and xylose. Fermentation of xylose is problematic. S. cerevisiae, which is currently responsible for 95% of all ethanol produced by fermentation, does not ferment this monosaccharide. Those organisms that do, are not ethanol tolerant and give poor ethanol yields. There are a number of possible ways by which S. cerevisiae could be employed in the alcoholic fermentation of xylose.
- Isomerization of the aldo-sugar, D-xylose, to the ketoform D-xylulose, which S. cerevisiae can ferment. This may be achieved by carrying out the yeast fermentation in the presence of a bacterial xylose isomerase.
- Genetic engineering of S. cerevisiae, to express genesfor either:
- A bacterial xylose isomerase, e.g. from species of Actinoplanes, Bacillus, etc.
- Xylose reductase and xylitol dehydrogenase from a pentose fermenting yeast, e.g. species of Candida, Pichia, etc. However, there are likely problems with cofactor imbalances with this option.
Z. mobilis has also been genetically engineered to ferment xylose and may play a future role in the production of ethanol from plant biomass, as may the genetically engineered E. coli mentioned earlier, and certain thermophilic microorganisms.
Reference and Sources
- https://www.agmrc.org/commodities-products/renewable-energy/cellulosic-ethanol
- https://link.springer.com/chapter/10.1007/978-981-16-3682-0_11
- https://pmc.ncbi.nlm.nih.gov/articles/PMC2651186/
- https://www.nature.com/articles/s43016-022-00589-6
- https://www.researchgate.net/publication/250672187_Biofuel_from_D_-xylose_-_The_second_most_abundant_sugar
- https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/elsc.201400199
- https://pubs.nmsu.edu/_circulars/CR612/index.html
- https://www.sciencedirect.com/science/article/pii/S1096717618300363
Also Read:
- Nitrogen Cycle
- Microbial Fuel Cells
- Enzymes used in textile, Leather and wood pulp manufacturing
- Prenatal Diagnosis- An Overview
- Nitrogen metabolism: Introduction and Overview
- Cancer: Intro, Types, Development and Therapy
- Bacterial vaccines – An Overview
- Molecular Detection of Microorganisms: Intro, Molecular methods, Applications
- Milk: Composition, Processing, Pasteurization, Pathogens and Spoilage
- SDS-PAGE: Introduction, Principle, Working and Steps
