Different types of Pathways for ATP Production
Introduction
- The catabolism of glucose is a technique to the metabolism of all organisms as it provides a glimpse of how organisms obtain energy for life.
- In this section, we examine how cells obtain energy from other organic compounds (fats and proteins) by directing those compounds into the process of cellular respiration.
- We also discover how modifications to cellular respiration and glucose metabolism allow anaerobic organisms to use glucose and generate ATP without having O2 as the final electron acceptor in electron transport.
Other Nutrients Represent Potential Energy Sources
A wide variety of organic compounds can serve as useful energy sources. All these compounds must go through a series of preparatory conversions before they are processed in glycolysis or the citric acid cycle.

Carbohydrates for ATP Production
- Besides glucose, other monosaccharides as well as disaccharides and polysaccharides can be used to produce ATP.
- Sucrose, for example, is first digested by the enzyme sucrase into its two monosaccharides, glucose and fructose.
- The glucose molecule gets into the glycolysis pathway directly, however the fructose molecule goes through further conversions and enters glycolysis as an intermediate further into the pathway.
- Lactose is an other disaccharide, which divided into glucose and galactose by help of the enzyme lactase.
- Galactose also undergoes a series of changes before it is ready to enter glycolysis.
- Stored polysaccharides, such as starch and glycogen, are metabolized by enzymes that remove one glucose unit at a time and through a series of chemical conversions are well prepared to enter the glycolysis pathway.
- Thus, carbohydrates other than glucose can be used as chemical energy sources.
Fats for ATP Production
- Fats are extremely valuable energy sources because their chemical bonds contain enormous amounts of chemical energy.
- Fats consist of three fatty acids bonded to a glycerol molecule.
- To be useful for energy purposes, the fatty acids are separated from the glycerol by the enzyme lipase. After this has taken place, the glycerol portion enters as an intermediate into glycolysis.
- For fatty acids, there is a complex series of conversions called beta-oxidation, in which each long-chain fatty acid is broken by enzymes into 2-carbon units.
- Other enzymes then convert each unit to a molecule of acetyl CoA that enters the citric acid cycle.
- We noted that the oxidation of one glucose molecule could generate 20 molecules of ATP in the citric acid cycle.
- A quick calculation should illustrate the substantial energy output from a 16-carbon fatty acid (i.e., eight 2-carbon units).
Proteins for ATP Production
- Although proteins are generally not considered energy sources, cells will use them when carbohydrates and fats are in short supply.
- Proteins are broken down to amino acids. Enzymes then convert many amino acids to energy pathway components by removing the amino group and substituting a carbonyl group. This process is called deamination.
- For example, alanine is converted to pyruvate and aspartic acid is converted to oxaloacetate.
- Consequently, in most habitats, microbes can use a large and diverse set of chemical compounds as potential energy sources.
Anaerobic Respiration Produces ATP Using Other Final Electron Acceptors

- Nearly all eukaryotic microbes as well as multicellular animals and plants carry out aerobic respiration, using oxygen as the final electron acceptor in the electron transport chain.
- However, many bacterial and archaeal organisms exist in environments in which oxygen is scarce or absent, such as in wetland soil and water, and within human and animal digestive tracts.
- In these environments, the organisms have evolved a respiratory process called anaerobic respiration that usually relies on an inorganic final electron acceptor other than O2 for ATP production.
- Considering the immense number of species that live in such anaerobic environments, anaerobic respiration is extremely important ecologically.
- Many human enteric, facultative species, for example, use nitrate (NO3–) with which electrons combine to form nitrite (NO2–) or another nitrogen product during electron transport.
- The obligate anaerobe Desulfovibrio uses sulfate (SO4–2) for anaerobic respiration.
- The sulfate combines with the electrons from the cytochrome chain, resulting in reduced hydrogen sulfide (H2S).
- This gas gives a rotten egg smell to the environment (as in a tightly compacted landfill).
- A final example is exhibited by the archaeal methanogens. These obligate anaerobes use CO2 or carbonate (CO3) as a final electron acceptor in the electron transport chain and, with hydrogen gas (H2), form large amounts of CH4.
- In anaerobic respiration, the amount of ATP produced is less than in aerobic respiration.
- There are several reasons for this. First, only a portion of the citric acid cycle functions in anaerobic respiration, so fewer reduced coenzymes are available to the electron transport chain.
- In addition, not all of the cytochrome complexes function during anaerobic respiration, so the ATP yield will be less.
- A slower ATP generation process means these organisms will grow slower than their aerobic counterparts, which generate more ATP per glucose.
Fermentation Produces ATP Using an Organic Final Electron Acceptor
- In environments that are depleted of oxygen gas (anoxic) and are lacking alternative final electron acceptors needed by anaerobes (e.g., NO3–, SO4–2, CO3), the pyruvate from glycolysis will be catabolized through fermentation.
- Fermentation, probably the most ancient form of energy metabolism, is the enzymatic process for producing ATP by substrate-level phosphorylation, using the organic compounds of glycolysis as both electron donors and acceptors.
- In fermentation, the citric acid cycle and oxidative phosphorylation processes do not function.
- The chemical process of fermentation makes many fewer ATP molecules than in cellular respiration.
- However, no matter what the end product, fermentation ensures a constant supply of NAD+ for glycolysis and the production of two ATP molecules per glucose.
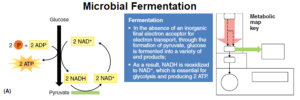
- In other words, the metabolic pathways for fermentation oxidize NADH to NAD+.
- For example, in the fermentation of glucose by Streptococcus lactis, the conversion of pyruvate to lactic acid is a way to reform the NAD+ coenzymes that glycolysis needs to continue generating two ATP molecules for every glucose molecule consumed.
- The diversity of fermentation chemistry extends to some eukaryotic microbes, as well.
- In yeasts like Saccharomyces, when pyruvate is converted to ethyl alcohol (ethanol), NAD+ is reformed and used for the continuation of glycolysis.
- The energy benefits to fermentative organisms are far less than in cellular respiration.
- In fermentation, each glucose passing through glycolysis yields two ATP molecules and the production of different final products depending on the microbial species.
- This is in sharp contrast to the 32 molecules from cellular respiration.
- It is clear that cellular respiration is the better choice for energy conservation, but under anoxic conditions, there might be little alternative if life for S. lactis, Saccharomyces, or any fermentative microorganism is to continue.
Reference and Sources
- https://www.slideshare.net/mimiian/energy-metabolism-16565080
- https://epdf.pub/alcamos-fundamentals-of-microbiology-ninth-edition.html
- https://quizlet.com/125213981/microbiology-lecture-exam-2-flash-cards/
- https://quizlet.com/193831206/microbiology-block-2-part-a-flash-cards/
- https://study.com/academy/lesson/anaerobic-respiration-definition-equation-examples.html
- https://quizlet.com/123026247/biol-1011-exam-2-flash-cards/
- https://www.researchgate.net/publication/267872932_Community_Composition_of_Known_and_Uncultured_Archaeal_Lineages_in_Anaerobic_or_Anoxic_Wastewater_Treatment_Sludge
- https://www.thoughtco.com/aerobic-vs-anaerobic-processes-1224566
- https://ecampusontario.pressbooks.pub/biology/chapter/4-5-connections-to-other-metabolic-pathways/
- https://www.slideshare.net/nitinyadav16/anaerobic-treatment-of-industrail-wastewater
Also Read:
- Microbial Fuel Cells
- The Genetic Code
- Biogeochemical Iron Cycle
- Biogeochemical Iron Cycle
- Transposable Elements
- Downstream processing and its steps
- Overview of Viroids, Satellites and prions
- AIDS: Acquired Immune Deficiency Syndrome
- Microorganisms in Benthic Marine Environments
- Exposure and Transmission of Infectious Disease
- Microorganisms in Benthic Marine Environments
- Innate Immunity: Description, Functions and Facts
- Proteomics: Introduction, Methods, Types and Application

Very informative