Proteins: Definition, Roles, Functions and Structure
Structure of Proteins
Proteins are composed of carbon, hydrogen, oxygen, nitrogen and usually, sulfur atoms. They are the most abundant organic compounds in all living organisms making up about 58% of a bacterial cell’s dry weight. this high percentage of protein indicates the essentials and diverse roles for these organic compounds.
Here are some of the roles for proteins:
- Structural proteins: Numerous proteins function as structural components of cells and cell walls.
- Transport proteins: Many proteins form channels or pores through membranes to facilitate the movement of materials into or out of the cell.
- Regulatory proteins: Some proteins bind to DNA and help regulate metabolic activity by switching genes on or off.
- Receptor proteins: Other proteins in cells act as sensors (receptors) that recognize chemicals in the environment.
- Cell motility proteins: Many microbes contain flagella or cilia, which are protein-containing structures involved with cell movement.
- Immune system proteins: The human immune system uses many different proteins, such as antibodies, to defend against and eliminate invading pathogens.
- Enzymes A large number of proteins serve as enzymes, protein catalysts that selectively speed up metabolic reactions.
Proteins are unbranched polymers built from nitrogen-containing monomers called amino acids. At the center of each amino acid is a carbon atom attached to two functional groups: an amino group (–NH2) and a carboxyl group (–COOH).
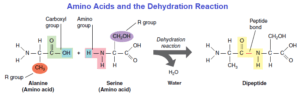
Also attached to the carbon is a side chain, called the R group (–R). Each of the 20 amino acids differs only by the atoms composing the R group. These side chains, many being functional groups, are essential in molding the final shape and function of proteins.
In forming a protein, amino acids (sometimes called peptides) are joined together by covalent (peptide) bonds where the carboxyl group of one amino acid is linked to the amino group of another amino acid through a dehydration reaction. Peptide (covalent) bonding between hundreds of amino acids produces a long polymer called a polypeptide. Because proteins have tremendously diverse roles, they come in many sizes and shapes. The final shape depends on the sequence of amino acids in the polypeptide.
Primary Structure of protein
The linear sequence of amino acids in a polypeptide represents the primary structure. Each protein that has a different function will have a different primary structure, as each position along the chain will have one of the 20 amino acids. The sequence of the amino acids is determined by the sequence of bases (genes) in the DNA. However, the sequence of amino acids alone is not sufficient to confer function to a protein.
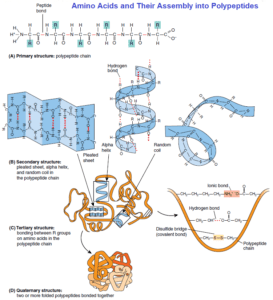
Secondary Structure of protein
Most proteins have regions folded into a corkscrew shape called an alpha helix. These regions represent part of the protein’s secondary structure. Hydrogen bonds between amino groups (–NH) and carbonyl groups (–CO) on nearby amino acids maintain this structure. A secondary structure also will form when the hydrogen bonds cause portions of the polypeptide chain to zigzag in a flat plane, forming a pleated sheet. Other regions of the protein might not interact and remain in a “random coil.”
Tertiary Structure of protein
Many proteins also have a tertiary structure superimposed on the secondary structure. Such a three-dimensional (3-D) shape of a protein is folded back on itself much like a spiral telephone cord. Ionic and hydrogen bonds between nearby R groups on amino acids help form and maintain the tertiary structure. In addition, covalent bonds, called disulfide bridges, between sulfhydryl (–SH) functional groups in R groups are important in stabilizing tertiary structure.
The ionic and hydrogen bonds helping hold a protein in its 3-D shape are relatively weak associations. As such, these interactions in a protein are influenced by environmental conditions. When subjected to heat, pH changes, or certain chemicals, these bonds can break, causing the polypeptide to unfold and lose its biological activity. This loss of 3-D shape is referred to as denaturation.
For example, the white of a boiled egg is denatured egg protein (albumin) and cottage cheese is denatured milk protein. Should enzymes be denatured in living cells, the important chemical reactions they facilitate will be interrupted and death of the cell or organism might result.
Viruses also can be destroyed by denaturing the proteins that build the virus’ 3-D structure. So, now you should understand the importance of buffers in cells; by preventing pH shifts, they prevent protein denaturation and maintain protein function.
Quaternary Structure of protein
Many proteins are single polypeptides. However, other proteins contain two or more polypeptides to form the complete and functional protein; this association of polypeptides is called the quaternary structure. Each polypeptide chain is folded into its tertiary structure and the unique association between separate polypeptides produces the quaternary structure. The same types of chemical bonds are involved as in tertiary structure.
Reference and Sources
- https://courses.lumenlearning.com/boundless-ap/chapter/proteins/
- https://bio.libretexts.org/Courses/University_of_California_Davis/BIS_2A_(2018)%3A_Introductory_Biology_(Singer)/Bis2A_Winter_2019/Lecture_04%3A_Biomolecules
- https://courses.lumenlearning.com/sanjacinto-biology1/chapter/proteins/
- http://mol-biol4masters.masters.grkraj.org/html/Cell_And_Molecular_Immunology1-Cells_And_Tissues_Of_Immune_System.htm
- https://quizlet.com/565063262/microbio-unit-1-flash-cards/
- https://findanyanswer.com/how-do-disulfides-stabilize-protein-structures
Also Read:
- Syphilis: Agent, Epidemiology, Symptoms, Treatment and Prevention
- Mutations: Introduction, Types, Causes and Repair Mechanisms
- DNA Replication in eukaryotes: Initiation, Elongation and Termination
- Proteomics: Introduction, Methods, Types and Application
- Transcription in prokaryotes: Initiation, Elongation and Termination
- Chromosomes: Structure, Morphology, Composition and Organization
- Histones types and its functions
- Tools of Genetic Engineering to Make Genome Modifications
- Microbial Identification and Strain Typing Using Molecular Techniques
- Whole-Genome Shotgun Sequencing: overview, steps and achievements
- Plasmid: Properties, Types, Replication and Organization
