Chromatography is a separation technique used to isolate, identify, and purify the components of a mixture. It is based on differences in how substances distribute between a mobile phase (moves) and a stationary phase (stays fixed).
- Chroma– “color” and graphein – “to write”.
- Chromatography is a technique in which substance are separated, purified and identified from a mixture for qualitative and quantitative analysis. On the basis of hydrophobic interactions, Polarity, enzymes and net charges are separated by using chromatography.
- Chromatography is a physical method of separation of compound.
- TSWET, referred as the father of chromatography.
- This technique was first used by Tswet, to separate various plants pigments such as chlorophylls and xanthophylls by passing these pigments through glass column packed with finely divided calcium carbonate.
Purpose of chromatography
- Analytical: To determine chemical composition of a sample.
- Preparative: Used in purification of a substance.
Principle of chromatography
- The principle of chromatography is very simple.
- The sample to be examine or analyzing is termed as solute or analyte is allowed to interact or react with two immiscible phases-
- Mobile phase
- Stationary phase
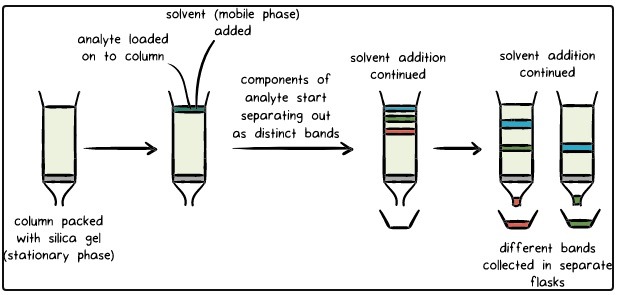
- The stationary phase which may be a solid is or a liquid supported on a solid, which is immobile it means it does not move.
- The mobile phase migrates the sample through stationary phase.
- The mobile phase may be either liquid or gas.
- All chromatographic methods involve separation of mobile phase through a stationary phase.
Classifications of chromatography
- Chromatography technique can be explained into three fundamental ways:
- Based on the shape of chromatographic bed.
- Based on the physical nature of the stationary and mobile phases.
- Based on the mechanism of the separation.

Based on the shape of chromatographic bed:
- On the basis of the chromatographic bed, there are following two types of chromatography-
- Planar chromatography.
- Column chromatography.
- In planar chromatography, the stationary phase is spread on a flat, planar surfaces. The plane can be paper acting as a stationary phase (paper chromatography), or stationary phase spread on glass, metal or plastic plate (thin layer chromatography). Planar chromatography also known as open-bed chromatography.
- In column chromatography, the stationary phase is within a tube.

Based on the physical nature of the mobile and stationary phase:
- On the basis of physical nature of mobile phase, there are two types of chromatography:
- Gas chromatography
- Liquid chromatography
- Further, on the basis of stationary phase, physical nature there are the following types:
- Gas-solid chromatography
- Gas-liquid chromatography
- Liquid-solid chromatography
- Liquid-liquid chromatography
Based on the mechanism of separation:
- The method of chromatography uses various types of mechanisms to separate analyte.
- On the basis of mechanism of separation, it is of following types:
- Partition chromatography
- Adsorption chromatography
- Size exclusion chromatography
- Affinity chromatography
- Ion exchange chromatography
Application of Chromatography
In the pharmaceutical sector:
- In detection of presence of trace elements or chemicals.
- This is also used for the separation of compound on the basis molecular weight and composition of elements.
- In the detection of unknown compound and in the purity of mixtures.
- In the development of drug.
In the Chemical industry:
- HPLC and GC are very much used for detecting various contaminants such as polychlorinated biphenyl (PCBs) in pesticides and oils.
In the Food Industry:
- In the detection of food spoilage and additives.
- In the quantitative and qualitative analysis of nutrients in food.
In Forensic Science:
- In forensic science, this technique is used in the analysis of crime scenes like hair and blood samples.
In Molecular Biology Studies:
- Various techniques in chromatography such as EC-LC-MS are involved in the study of metabolomics and proteomics along with nucleic acid research.
- HPLC is used in Protein Separation like Insulin Purification, Plasma Fractionation, and Enzyme Purification and also used in the various industries like Fuel Industry, biochemical processes and biotechnology.
Chromatography like HPLC is used in DNA fingerprinting and bioinformatics:
- Assay & Content Uniformity.
- HPLC in fingerprinting and Bioinformatics.
- Petrochemicals and Catalysis.
- Ebola Immunization.
- Polymer Synthesis.
- Clinical diagnosis of diseases and disorder.
- LC-NMR (liquid chromatography (LC) and nuclear magnetic resonance).
Reference and sources:
- https://microbenotes.com/chromatography-principle-types-and-applications/
- https://chromatography.conferenceseries.com/events-list/applications-of-chromatography
- https://www.researchgate.net/publication/310994699_Fundamentals_and_Techniques_of_Biophysics_and_Molecular_Biology

5 thoughts on “Chromatography: Introduction, Principle, Classification and Applications”
clearly explained, definitely easier than how school explains it. so helpful.
It’s very much good web for study
This post on chromatography was incredibly informative! I appreciated the clear explanation of the principles and the various classifications. The applications section really opened my eyes to how widely this technique is used in different fields. Looking forward to more in-depth posts on specific chromatography methods!
This blog post is a fantastic introduction to chromatography! I appreciate how clearly you’ve explained the principles and different classifications. The applications section was particularly helpful in understanding its real-world relevance. Looking forward to more posts like this!
It is really an amazing website for students who are looking for best notes 👏 highly knowledgeable, I will recommended it to my all friends