Biosafety cabinet: Introduction, development and safety
Introduction
While appalling today, it was not long ago when a visitor would see mouth pipetting, smoking, and eating in the microbiology laboratory. Over the years, several key events have slowly but quite significantly changed the laboratory culture to promote safety.
Development of biosafety cabinet
Biosafety cabinet developed by Arnold G. Wedum, M.D., Ph.D., at the Army Biological Research Laboratory, Fort Detrick, Maryland, during the 1950s and 1960s set a standard for physical containment strategies and best practices to keep microbiologists from acquiring laboratory infections.
Wedum established these strategies while working with some of the world’s most deadly infectious agents being studied as part of the U.S. biological warfare program. The development of offensive biological weapons was terminated in 1970 by order of President Richard Nixon. Defensive counter measures continued to be studied at Fort Detrick, fostering numerous additional methods for the safe manipulation of microorganisms.

Later, the ground-breaking methods for manipulating DNA by Paul Berg in the 1970s sparked an international discussion about the possible hazards of recombinant DNA technology. The resulting Asilomar Conference on Recombinant DNA in 1975 set a requirement for the systematic evaluation of safe practices in the laboratory.
The Need for Safety Guidance
Subsequently concern over standards needed to safely study cancer-causing viruses prompted the National Institutes of Health to establish criteria for ascending levels of physical containment and specific procedures to
prevent human contamination. It was 10 years later when these best methods and work practices were assembled into one “manual of biosafety guidance.”
This was published in 1984 as Biosafety in Microbiological and Biomedical Laboratories, or BMBL. The fifth and current edition of the BMBL was published in 2007 and updated in 2010. Importantly, the editors insightfully prefaced the fifth edition with the caveat that “the intent was and is to establish a voluntary code of practice, one that all members of a laboratory community will together embrace to safeguard themselves and their colleagues, and to protect the public health and environment.”
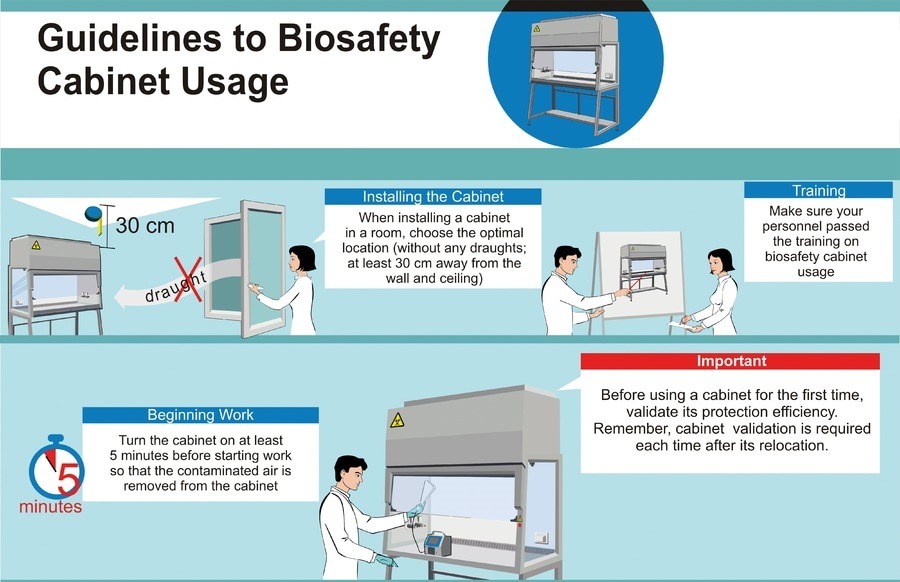
To help prevent laboratory-acquired infections (LAIs), microbiologists today follow strict procedures, often called standard microbiological practices, based in the thoughtful work by Wedum, Berg, and countless others, and codified in the BMBL. In addition, some events have led to the formation of laws affecting how microbiologists think about and practice laboratory safety and security.
For example:
- The recognition that the human immunodeficiency virus (HIV) and other viruses were transmitted by blood led to the 1991 Blood borne Pathogens Act.
- The use of anthrax as a bioterrorism agent led to the Public Health Security and Bioterrorism Preparedness and Response Act of 2002.
- The response to emerging viruses with high infectivity or high lethality (such as SARS and avian influenza) resulted in the 2005 Pandemic and All-Hazards Preparedness Act. Regardless of the reason, the message is clear: safety in the microbiology lab must be a high priority.
The standard microbiological practices that guide lab safety
Safety planning for the microbiology laboratory should focus on a risk assessment process where one evaluates the likelihood of a LAI in light of the potential consequences of infection. Inherent in this assessment is not just the potential to cause disease in the user but also the potential for contamination beyond the laboratory.
Typically, the laboratory design (e.g., airflow, sink access, decontamination processes) and access to biosafety cabinets (if used) are surveyed so as to remedy the hazard or at least identify it for the laboratory users.

Following this initial survey, potential risks associated with manipulation of microorganisms are considered, including:
- Infectious agent risk group.
- Procedure used with each agent.
- Experience of those using the laboratory.
There are four risk groups (RGs) into which pathogens are assigned. These RGs reflect increasing danger to humans and are determined based on a number of criteria, including pathogenicity, virulence factors, mode of transmission, availability of protective vaccines and chemotherapeutic agents, and DNA content beyond wild type, among others.
The rest of the risk assessment is to determine the biosafety level (BSL). The BSL designation indicates that a particular risk is acknowledged and that a specific set of work practices, safety equipment, and facility engineering controls will be used in an assigned laboratory space to keep risk low.

The four biosafety levels reflect an appropriate containment level used to prevent LAI and environmental contamination. Each subsequent level builds on the previous. The common component to all levels is the foundational standard microbiology practices, encompassing the behaviors directly under the user’s control. These represent minimum guidelines for laboratory safety.
In addition to accepted laboratory behaviors, specific pieces of safety equipment are often recommended for each BSL. Personal protective equipment (PPE) is a unique combination of safety equipment designed as body protection.
PPE includes:
- Protective coverings (coats, gowns, or uniforms).
- Protective eye wear
- Gloves (latex and nonlatex options).

PPE should prevent transmission of infectious agents by blocking their access to entry portals. Additional PPE (e.g., face shield, respiratory protection) may be necessary as determined by the risk assessment.
Reference and Sources
- https://www.cdc.gov/mmwr/preview/mmwrhtml/su6101a1.htm
- https://silo.pub/dna-the-secret-of-life.html
- https://quizlet.com/87675301/lecture-11-clinical-microbiology-and-immunology-flash-cards/
- https://en.wikipedia.org/wiki/Statement_on_Chemical_and_Biological_Defense_Policies_and_Programs
- https://medium.com/lsf-magazine/the-invention-of-recombinant-dna-technology-e040a8a1fa22
- https://history.nih.gov/display/history/Wilson%2C+Samuel+2004
- https://en.wikipedia.org/wiki/Editions_of_Dungeons_%26_Dragons
Also Read:
- Nitrogen Cycle
- Anti protozoan Drugs
- The Genetic Code
- Standard Operating Procedures or SOP
- Overview of an antimicrobial agents
- DNA replication in prokaryotes
- Vector: properties, types and characteristics
- Cider: Production, Extraction, Fermentation and Maturation
- Roles of Viruses In Aquatic Ecosystems
- Milk: Composition, Processing, Pasteurization, Pathogens and Spoilage
- Probiotics: Introduction, Development and Uses in Agriculture
- CRISPR-Cas9 Gene editing tool: Introduction, Principles, Uses & Applications
- Microorganisms in Benthic Marine Environments
