Spectroscopy and its Types
Definition
- Spectroscopy is the collective terms for diverse group of techniques which involves the interactions of electromagnetic radiation with a matter of interest are studied.
- Radiation is either get scattered, emitted or absorbed, when the radiations meets with the matter.
Instruments
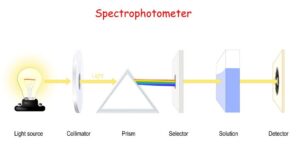
- An instrument which is used to measure the absorbance by measuring the amount of light of a given wavelength that is transmitted by a sample is termed
- A spectrophotometer mainly consists of a monochromator, a light source, sample holder(cuvette) and a light detector.
- The spectroscopic technique is mainly used for the determination of concentration or amount of a given species.
Types of spectroscopy
- Based on this there is three principles branches of spectroscopy.
- Absorption spectroscopy
- Scattering spectroscopy
- Emission spectroscopy
- Absorption spectroscopy uses the range in which a material absorbs the electromagnetic spectrum. It involves atomic absorption spectroscopy and different molecular techniques that area, such as infrared spectroscopy and radio region nuclear magnetic resonance (NMR) spectroscopy.
At selected wavelength, radiation’s intensity is attenuated absorption E.g.- X-ray, UV, IR.
- Emission spectroscopy uses the electromagnetic spectrum range in which a material radiates (emits). The material must consume energy first. This energy may come from several sources, such as luminescence, which defines the subsequent emission. Spectro fluorimetry includes molecular luminescence techniques.
Amount of light that a substance scatters at certain wavelength E.g.- Raman spec.
- Scattering spectroscopy tests the amount of light at specific wavelengths, incident angles, and polarization angles that a material scatter. The method of scattering is much quicker than the process of absorption/emission. Raman spectroscopy is one of the most beneficial applications of light scattering spectroscopy.
Fluorescence- stokes shift- difference between the absorption and emission wavelength is called stokes shift.
UV/VIS absorption spectroscopy
- Transition of electrons from one molecular orbital to other due to absorption of electromagnetic radiation (UV/vis).
- Transitions occurs from highest occupied orbital (π) to lowest unoccupied orbital (σ),
Single bond between C-H, O-H- (σ).
Multiple bonds C=C, C=N (π).
- The UV light is absorbed by various compounds, namely by those having conjugate double bonds. Both proteins and nucleic acids absorb strongly UV light, which can be used for their investigations.
- Some amino acids such as tryptophan and tyrosine have absorption maximum at about 280 nm. Phenylalanine at 255 nm.
- Nucleotides have absorption maximum in the range of 260-270 nm.
- Chromophores– there is variation in the absorption properties according to chemical composition of the medium.
- Molecules containing functional groups, capable of absorbing UV/VIS radiation are called chromophores.
There are three types of chromophores in proteins which is relevant for UV/Vis spectroscopy:
- Peptide bonds or amide bond
- Some amino acid side chains (mainly tryptophan and tyrosine); and
- Some prosthetic groups and coenzymes e.g., porphyrins groups.
IR spectrophotometry
- Infrared can interact with rotational and vibration states of molecules.
- There are different modes and ways through which Complex molecules can vibrate or rotate. Various chemical groups (-CH3, -OH, -COOH, -NH2) have specific vibration and rotation frequencies and thus absorb IR light of specific wavelength.
- Therefore, infrared absorption spectra have many maxima. A change in chemical structure is exhibit as changes of the position of these maxima.
- It is also known as vibrational spectroscopy because it causes vibrational transitions.
- The vibrations in the IR spectroscopy are termed as fundamental vibrations.
- IR spectrum is most commonly used for structural analysis to determine the functional groups.
- Wave number of the infrared region of the spectrum which ranges from about 12800 to 10 cm-1 .
Nuclear magnetic resonance (NMR)
- NMR involves change in nuclear spin energy in presence of external magnetic field.
- Absorption of EMR in radio frequency, protons, electrons, and neutrons possess property of spin.
- Spin: ½ (+ or -) spin angular momentum (p+n).
- In NMR machine magnetic field is given
- Atoms will arrange in that magnetic field directions, if H is surrounded by other atoms, electrons give shield to this proton, so they are not oriented as per directions of magnetic field set by NMR.
Raman spectroscopy
- Scattering is a physical process which may causes the radiations to deviate from a straight trajectory.
- In elastic scattering of monochromatic light-Raman spectroscopy scattered photons are shifted in frequency.
- Elastic scattering or Rayleigh scattering; light which encounters the molecules in air, it is predominant mode of scattering.
- Any change in the energy of the scattered photon corresponds exactly to the photon energy as a result, the wavelength of the scattered photons can be longer (stokes raman scattering) or shorter (anti-stokes Raman scattering).
Reference and Sources
- https://microscopiaiwm.com/2021/03/19/spectroscopy/
- https://pubs.rsc.org/en/content/articlehtml/2018/nr/c8nr02278j
- https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Book%3A_Analytical_Chemistry_2.1_(Harvey)/10%3A_Spectroscopic_Methods/10.02%3A_Spectroscopy_Based_on_Absorption
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/infrared-spectroscopy
- https://www.med.muni.cz/biofyz/files/biomedicina/Pristroje_molekularni_BF.ppt
- https://www.scribd.com/document/357596012/Biophysics-Molecular-Biology-pdf
- http://cms.gcg11.ac.in/attachments/article/107/Raman_pdf.pdf
Also Read:
- Types of microscopes
- Downstream processing and its steps
- Different types of Pathways for ATP Production
- Microscopy: Overview, Principles and Its Types
- Gel electrophoresis: types, principles, instrumentation and applications
- Biosafety Cabinet: Introduction, Development and Safety guidance
- Chromatography: Introduction, Principle, Classification and Applications

1 thought on “Spectroscopy and its Types ”