Carbohydrate: Structure, Functions and Types
- Carbohydrates are most abundant biomolecules in the nature.
- They can hydrolyze Polyhydroxy aldehyde, ketones, or compounds.
- Carbon, hydrogen, and oxygen are the primary component of carbohydrates.
- They are also known as hydrates of carbon because in most of the carbohydrates H and O are present in the same ratio as in water.
Functions
Carbohydrates are involved in various functions:
- They also act as precursor for many organic compounds.
- It also involved in structural components of many organisms.
- It also involves in immediate source of energy demands of the body by serving as the storage form of energy (glycogen).
- They also involved in the structural and cellular functions of cell such as- fertilization, cell growth and adhesion.
Classifications

- Carbohydrates are commonly referred to as a saccharides (greek: sakcharon means sugar).
- Carbohydrates are classified into three major groups depending upon whether these undergo hydrolysis and if so on then the number of products formed by them.
- Monosaccharides
- Oligosaccharides
- Polysaccharides
Monosaccharides
- Greek: mono means one.
- It consists of single polyhydroxy aldehyde or ketone unit.
- They are the simplest sugar and generally, referred as simple sugars.
- General formula is cnh2no
- They are colorless, crystalline solids that are soluble in water but insoluble in nonpolar solvents.
- They cannot further hydrolyzed to form simpler molecules.

- It is divided into two categories on the basis of functional groups:
-
- Aldoses: monosaccharides with aldehyde groups e.g. glyceraldehyde, glucose.
- Ketoses: monosaccharides with ketone groups e.g. dihydroxyacetone, fructose.
- D-glucose is the most abundant monosaccharides on the earth.
Structural aspects of carbohydrates
- One of the most important character of monosaccharides are stereoisomerism.
- Stereoisomerism may be defined as compounds which have same structural formula but differ in their spatial configuration.
- All the monosaccharides except dhap (Dihydroxyacetone phosphate) contains one or more asymmetric carbon atoms.
Enantiomers
- Chiral molecules can exist in two configurations that are not super imposable mirror images to each other.
- The two members are designated as d- and l- sugars.
Methamphetamine Enantiomers
Epimers
- Most of the sugars are closely related, but differs only by the stereo-chemistry at a single carbon atom.
- Example: D-glucose and D-mannose they are differ at only carbon number 2.

Anomers
- Cyclic structure exists in two different configurational forms. If the OH- groups in the anomeric carbon is below the plane of the ring, then it is termed as alpha position; if the OH- groups is above the plane of ring then they are termed as beta position.
- These two diastereomers are termed as anomers.
Derivatives of monosaccharides
There are various monosaccharides, which are physiologically important:
- Sugar acids.
- Sugar alcohols
- Alditols
- Amino sugars.
- Deoxysugars
- L-ascorbic acid
Oligosaccharides
- Greek: oligo means few.
- It contains 2-10 monosaccharides molecules which are liberated on hydrolysis.
- It can be further divided into several groups depending upon the number of monosaccharides units present in them.

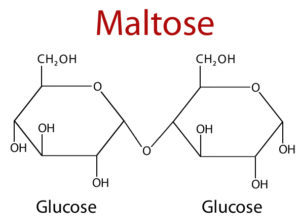
Disaccharides
- Disaccharides are the most common, among oligosaccharides.
- It consists of two monosaccharides units which is held together by glycosidic bond.
- It is crystalline, soluble in water and sweet in taste.
- It is of two types:
-
- Reducing; free aldehyde or keto group
- Non reducing: no free aldehyde or keto group.
Occurrence and biochemical roles of some important disaccharides:
| Disaccharides | Structure | Roles |
| Sucrose | Glucose (α1→2β) fructose | A product of photosynthesis. |
| Lactose | Galactose β(1→4) glucose | A major animal energy source. |
| Trehalose | Glucose α (1→1)α glucose | A major circulatory sugar in insects, used for energy. |
| Maltose | Glucose α(1→4) glucose | The dimer derived from the starch and glycogen. |
| Cellobiose | Glucose β(1→4) glucose | The dimer of cellulose polymer. |
| Gentiobiose | Glucose β(1-6) glucose | Constituent of plant glycosides and some polysaccharides. |
Polysaccharides
- Greek: poly means many.
- They are generally polymers of monosaccharide units with high molecular weight.
- They are tasteless and most often form colloids with water.
- It is linear as well as branched polymer.
- It is of two types:
-
- Homopolysaccharides: it yields only a single type of monosaccharide on hydrolysis.
- Heteropolysaccharides: it yields a mixture of a few monosaccharides or their derivatives on hydrolysis.
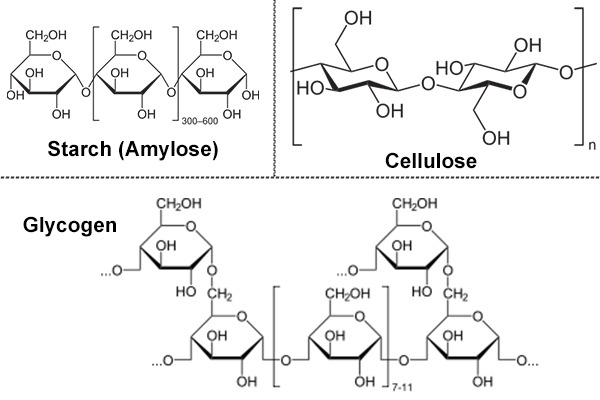
- Starch, glycogen, cellulose and chitin are homopolysaccharides.
- Glycosaminoglycan, peptidoglycan are heteropolysaccharides.
References and Sources:
- https://www.scribd.com/document/395829260/LifeSciencesPart-1-FifthEdition
- https://www.scribd.com/document/80796289/Carbohydrates
- https://www.slideshare.net/shefalijaiswal2/carbohydrates-91702638
- https://yourchemistrymaster.blogspot.com/2009/11/unit-17-biomolecules.html
Also Read:
- what is microbiology?
- What is Gene Expression?
- DNA replication in prokaryotes
- Deoxyribonucleic acid (DNA)
- Roles of Viruses In Aquatic Ecosystems
- Probiotics: Introduction, Development and Uses in Agriculture
- DNA Replication in eukaryotes: Initiation, Elongation and Termination
- Overview of lac operon an inducible operon
- Different types of Pathways for ATP Production
- Citric acid: Introduction, Fermentation, Recovery and Uses
- Mutations: Introduction, Types, Causes and Repair Mechanisms
- Cider: Production, Extraction, Fermentation and Maturation
- Amino acids: physical, chemical properties and peptide bond
- Biosafety Cabinet: Introduction, Development and Safety guidance
- Histoplasmosis: Symptoms, Pathogenesis, Treatment and Prevention
- Cold sores : Introduction, Entry, Symptoms, Diagnosis and Cure

i like what they tell me about the carbohydrate
They remind me of having a job