Amino acids: Physical, Chemical properties and Peptide bond
- Amino acids are group of group organic compound containing carbon, hydrogen, oxygen, and nitrogen.
- Amino acids are the building blocks or serve as monomers of protein.
- Proteins are the polymers of amino acids, with each amino acid residue joined by a specific type of covalent bond.
- As the name implies, it contains two functional groups- amino group and carboxyl groups.
- The amino group is basic while the carboxyl group is acidic in nature.
Properties of amino acid
The characteristics of proteins is ultimately depending upon the physical and chemical properties of amino acids.
Physical properties of Amino acids
- Solubility: Amino acids are most commonly soluble in water and insoluble in organic solvents.
- Melting points: Most of the amino acids are generally melt at higher temperatures, often above 200o.
- Taste: Gly, Ala, Val – sweet, Leu- tasteless, Arg lle- bitter.
- In various food industries Monosodium glutamate (MSG) and azinomoto is used as flavoring agent and widely used in Chinese foods to increase taste and flavor.
Optical properties of amino acids
- All amino acids except glycine, the alpha carbon atom is bonded to four different groups (a carboxyl group, an amino group, an R group and a hydrogen atom). Thus, the alpha carbon atom is a chiral center.
- All amino acids, which have a chiral center, are optically active which means they rotate the plane polarized light. An optically active compounds can rotate the plane polarized light either to the right (clockwise) or to the left (anticlockwise).
- Compounds that rotate the plane polarized light in clockwise direction are termed as dextrorotatory and designated by plus sign (+), while the compounds that rotate the plane polarized light in anti or counterclockwise are termed as levorotatory and designated by minus sign (-).
- Amino acids as ampholytes: The carboxyl and amino group of amino acids, along with ionizable R groups of some amino acids, are functions as weak acids and weak bases.
- Zwitterion or dipolar ion: (German for “hybrid ion”), it is hybrid molecule contains both positive and negative ionic groups. When an amino acid lacking ionizable R group is dissolved in water at neutral pH, it can exist in solution as the dipolar ion, or zwitterion which can acts as either an acids or bases.
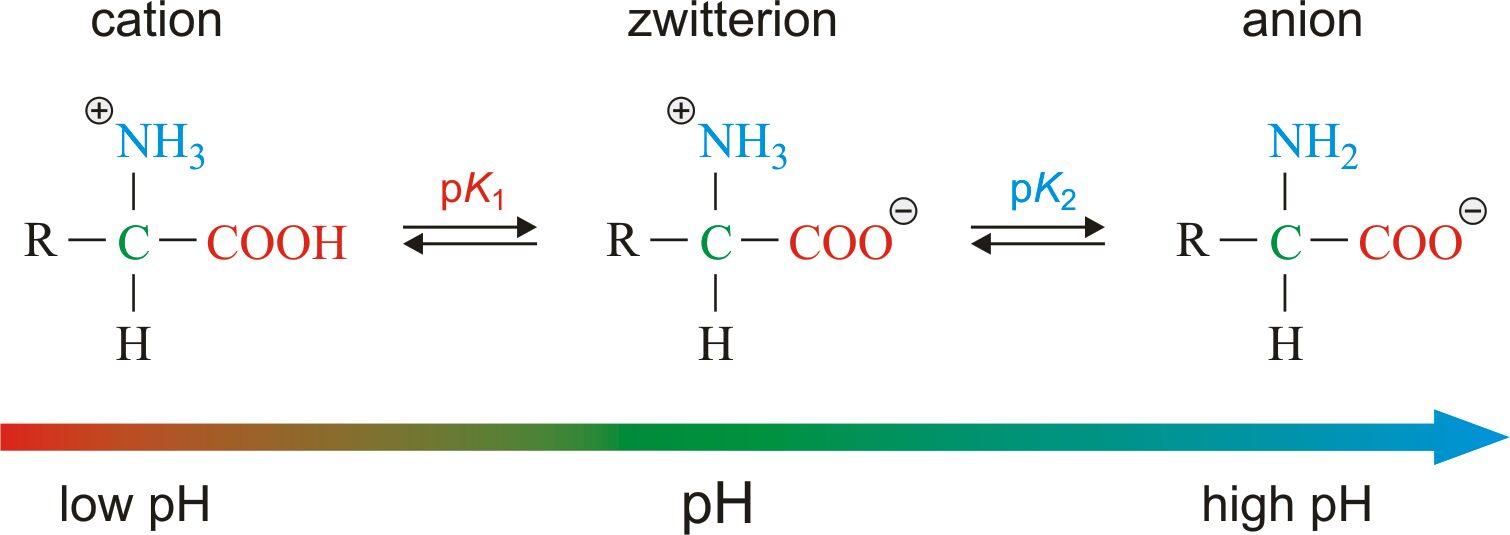
- Substance having this dual acid-base nature are amphoteric and are often called ampholytes. It is most rare that amino acid are exists in neutral form with free amino groups and free carboxylic group. Each amino acid has a characteristics pH at which it carries both the charges and acts as a zwitterion.
Chemical properties of amino acids
The general reactions of amino acids are most commonly due to the presence of two functional group viz. amino and carboxyl group.
Reaction due to -COOH group
- Amino acids commonly form esters (-COOR’) with alcohols, and salts (-COONa) with bases.
- Amnio acids produces amines through the process of decarboxylation.
- The carboxyl group of dicarboxylic amino acids reacts with amnio acids reacts with ammonia to form amide.
- Aspartic acid + NH3 ——-> asparagine
- Glutamic acid + NH3 ——-> glutamine
Reaction due to -NH2 group
- Amino group behaves as bases and it reacts with acids and produces salts.
- Reaction with Ninhydrin: The alpha amino acids react with ninhydrin and produces blue, pink or purple color complex.
- Amino acid + Ninhydrin ——> keto acid + NH3 + CO2 + Hydrindantin.
- Hydrindantin + NH3+ Ninhydrin ——> Ruhemann’s purple.
- Color reactions with amino acids: They are generally identified by specific color reactions.
- Transamination: One of the most important reaction in amino acids metabolism is transfer of amnio group from an amino acid to a keto acid to form a new amino acid.
- Oxidative deamination: It liberates free ammonia by the process of oxidative deamination.
Peptide bond
- The peptides and proteins are biologically occurring polypeptides that ranges from very small size to very large size, consisting of 2 or 3 to thousands of amino residues.
- A substituted amide linkage, termed as peptide bond to yield a dipeptide, can covalently hold two amino acids together.
- These peptide bonds are rather strong and generally serves as the cementing material between the individual amnio acids.
Formation of peptide bond
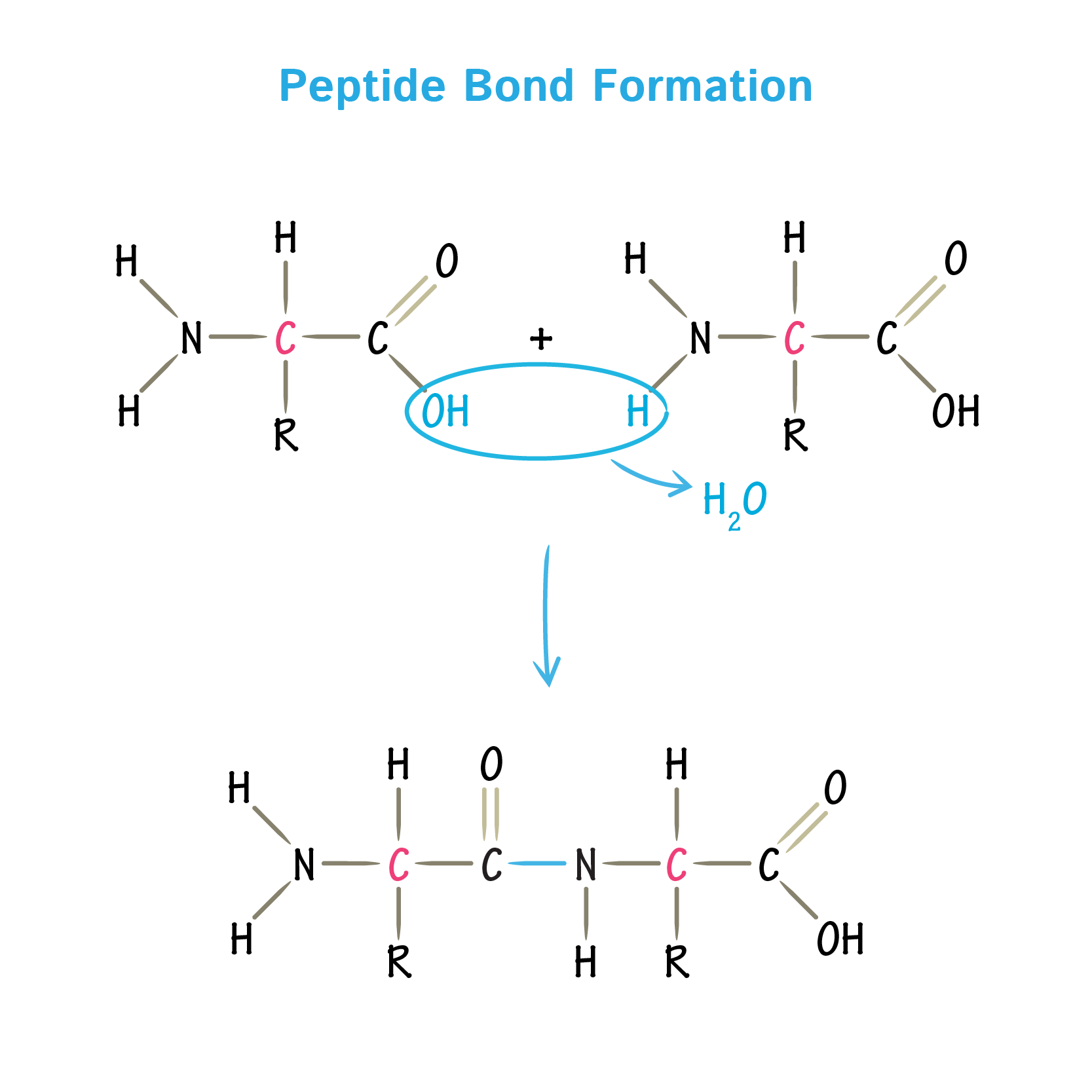
- This type of bonds is formed when the amino group of one amino acid combines with the carboxyl group of another amino acid.
- Such a linkages is formed by the removal; of elements of water or by dehydration from the alpha amino group of one amino acid and the alpha carboxyl group of another.
- Formation of peptide bond is an example of condensation reaction, a common class of reactions in living cells.
- Two amino acids can be joined by one peptide bond to form dipeptide.
- Three amino acids can be joined by two peptide bonds to form a tripeptide; similarly, four amino acids can be joined by three peptide bonds to form tetrapeptide, five to form a pentapeptide, and so forth.
- When few amino acids can be joined in this pattern, the structure is termed as oligopeptide while, many amino acids are joined, the product is called a polypeptide.
Characteristics
- It is rigid and planar, with partial double in nature.
- It most commonly occurs in trans configuration.
- In peptide bond, both -C=O and -NH groups are polar in nature and are involved in the formation of hydrogen bond.
Writing of peptide structure
- Conventionally, the peptide chains are written with the free amino end (N terminal residue) at the left and the free carboxyl end (C- terminal residue) at the right.
- The amino acids sequence can be read from N- terminal to C- terminal end.
- Incidentally, protein biosynthesis also begins from the N-terminal of amino acid.
References and Sources:
- https://upendrats.blogspot.com/2014/06/the-amino-acids.html
- https://www.slideshare.net/SciAmany/protein-chemistry-82362603
- https://biochemden.com/peptide-bonds/
- https://www.slideshare.net/Sadeesha_Fernando/bio-chemistry-78082793
