Starch processing enzymes and related carbohydrases
Microbial enzymes have proved to be of immense value in the processing of starch, a polysaccharide composed of amylose (linear α-1,4-linked glucose units) and amylopectin (branched polymer with both α-1,4 and α-1,6 linkages). α-Amylase (1,4-α-d-glucan glucanohydrolase) is one of the most important of these industrial enzymes. This endo-enzyme acts randomly on α-1,4 linkages, and ultimately generates glucose, maltose and maltotriose units. It can also catalyse the cleavage of internal α-1,4 linkages in glycogen and oligosaccharides.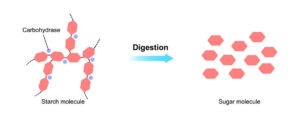
A further success has been amyloglucosidase (glucoamylase) from Aspergillus niger and Rhizopus species, which first became available in the early 1960s. This enzyme can completely break down starch and dextrins into glucose. Within a few years of its introduction, almost all conventional glucose production via acid hydrolysis was replaced by enzyme-based processes, because of the greater yield, higher degree of purity and easier product crystallization. The process was further improved by using highly thermostable bacterial amylase in starch pre-treatment (liquefaction).
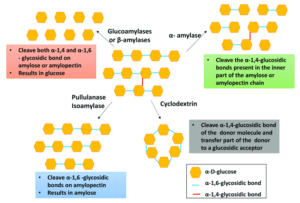
Glucose isomerase has also been a notable success in the starch processing industry. This enzyme can be obtained from many bacteria, including species of Bacillus and Streptomyces, and is usually immobilized for use in the conversion of glucose to fructose. The product, where conversion to fructose is completed, has the same calorific value as glucose, but its sweetening effect is approximately twice as high. Substrate glucose is normally derived from hydrolysed corn starch, and is then converted to a mixture of glucose and fructose by glucose isomerase. Practical conversion yields are in the range of 40–50% and the products are referred to as ‘high fructose corn syrup’ (HFCS) or ‘isosyrup’.
Fructose concentration can be increased to 55% by chromatographic enrichment. Alternatively, the fructose can now be separated from the glucose–fructose mixture and the remaining glucose fraction is isomerized to produce a final combined product containing up to 80% fructose. These syrups, derived from starch, are somewhat cheaper than sucrose from cane or beet sources, but have the same sweetening power. Consequently, they have become major food ingredients, particularly in North America, and annual production of HFCSs is now in excess of 8×109 kg.
Invertase (β-fructofuranosidase) was the first enzyme to be immobilized for use on an industrial scale. It was developed in the UK by Tate and Lyle during the early 1940s for syrup production. The conversion of sucrose to glucose and fructose using this immobilized invertase replaced conventional acid hydrolysis methods. This enzyme was originally prepared from autolysed S. cerevisiae preparations, which were adjusted to pH 4.7, clarified by filtration through calcium sulphate and then adsorbed onto bone char.
Invertase is now also obtained from other yeasts or filamentous fungi, e.g. A. niger and A. oryzae. Apart from syrup production, it is employed in confectionery manufacture. For example, softcentred chocolates are prepared by taking a solid sucrose- based filling, containing some invertase, and coating with chocolate. Within 2 weeks the centre becomes converted into a fructose/glucose syrup.
Lactase (β-galactosidase) is employed in several industrial processes that require the hydrolysis of lactose from milk, where the disaccharide is present at a concentration of 4.7% (w/v). Hydrolysis of lactose to glucose and galactose is performed on milk and milk products for infants who are lactose-intolerant, and in regions of the world where a large proportion of the adult population exhibit lactose-intolerance, e.g. regions of Africa and South-East Asia.
Lactose hydrolysis is also useful in the manufacture of products such as ice cream, as the low solubility of this disaccharide leads to crystal formation that may give an unpleasant sandy texture. In addition, its conversion to glucose and galactose increases the relative sweetness by about four-fold.
Commercial lactase is obtained from Kluyveromyces marxianus (formerly K. fragilis), A. niger or A. oryzae. The yeast enzymes have pH optima of 6.0–7.0, whereas the Aspergillus enzymes have optima as low as pH 3.0–4.0. These enzymes may be used free or immobilized, the latter being particularly useful for whey treatment. Yeast enzymes have been immobilized by incorporation into cellulose acetate fibres and the Aspergillus enzymes have been immobilized on 0.5mm diameter porous silica for use in packed bed reactors.
Reference and Sources:
- https://www.sciencedirect.com/science/article/pii/S0260877405003584
- https://www.sciencedirect.com/science/article/pii/S0147651315001232
Also Read:
- Virology: Introduction, Virus classification and Viral diseases
- Overview of Glucose Catabolism via Glycolysis
- Different types of Pathways for ATP Production
- Text and Practical Microbiology for MLT
- Fermentation: Introduction and its Types
- Carbohydrate: Structure, Functions and Types
- Beer brewing process


1 thought on “Exploring the Role of Starch Processing Enzymes in Carbohydrate Breakdown”
Amazing notes