Introduction
Citric acid is a weak organic acid that occurs naturally in citrus fruits such as lemons and oranges. It plays a central role in cellular metabolism, particularly in the Krebs cycle (Citric Acid Cycle or TCA Cycle), which is essential for energy production in aerobic organisms.
- It is a weak organic acid and the chemical formula is C₆H₈O₇.
- It also occurs naturally as a component of the many fruits.
- Citric acid is also produced by a fungal (fungi) fermentation.
- In biochemistry, it is an intermediary in the citric acid cycle, that occurs in metabolism reactions of all types of aerobic organisms.
- It is type of organic acids which accumulates during the controlled fermentative growth of particular species of Penicillium and Aspergillus.
MICROORGANISMS
These are Microbes which are most commonly used in the production of citric acid:
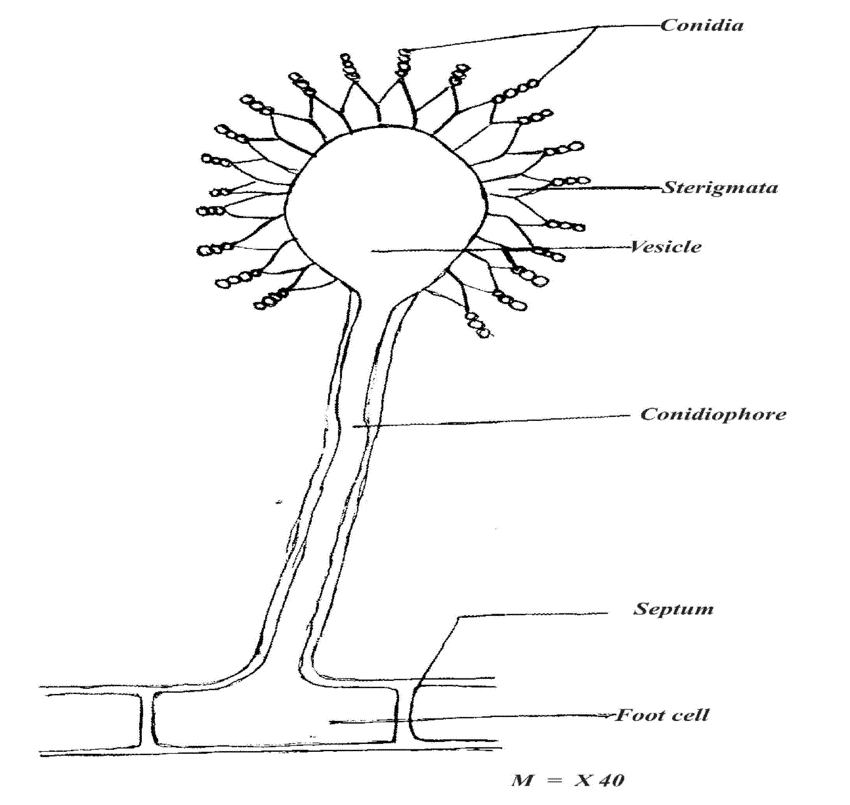
Eg.- Aspergillus niger and Candida yeast.
INOCULUM
- Strains of Aspergillus is selected for positive characteristics of citric acid yield, amount of sporulation, strain stability and so forth.
- For the negative characteristics of lack of ability to degrade the citric acid product, and of lack of concurrent formation of other acids such as oxalic, gluconic, and 5ketogluconic.
- Once strain has been selected, it is carefully watched during stock culture maintenance for culture degeneration.
- Various procedures of stock culture maintenance have been employed but, overall the cultures are best stored in the form of dry spores.
MEDIUM
- Media requirement for high production:
- Carbon source: beet molasses or sugar solution.
- Na- Ferro cyanide is added to reduce iron (1.3 ppm), manganese (<0.1 ppm).
- High dissolved oxygen concentration.
- High sugar concentration.
- The lower ph value at the time of production phase (ph <2) which minimize the risk of spoliation by different organisms and inhibits the manufacturing of unwanted organic acids.
- Temperature: 30 ˚C
- Nitrogen and phosphate limitations: Few complex media are rich and rarely need to be supplemented with a nitrogen.
- Trace elements: Zn, Mn, Fe, Cu and heavy metals.
- Bioreactors: batch or fed batch.
- Aeration: Is provided to the fermenters by air sparging.
- Agitation: Is to avoid shear damage on molds.
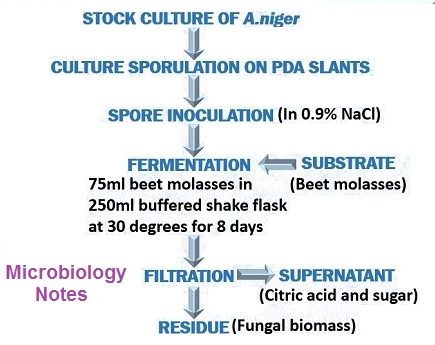
FERMENTATION
- Strains of, A.niger therefore are carefully selected for the positive characteristics of citric acid.
- Once a strain has been selected, it is carefully watched during stock culture maintenance for culture degeneration.
- The selection of a favorable fermentation medium probably is the most critical factor is obtaining high level accumulation of citric acid by A.niger.
- The medium should be slightly deficient in phosphate or in one or more metals and Cu.Mn, Zn, Fe.
- It has been found that methanol adds to the medium at slightly toxic level increases tolerance of the fungus to Zn, Mn, Fe.
- A beet molasses medium containing in the range of 10-20% sugar.
- Ammonium nitrate, magnesium sulphate and K2HPo4 are added.
- HCl is added to adjust the medium to a low, ph.
- Submerged aeration.
- Technique or utilizing stationary pans or trays containing shallow layers of medium.
- 28 to 30˚C with proper aeration.
RECOVERY
- Firstly, it involves the removal of fungal mycelium from the culture medium.
- Then, filter to remove mycelia and precipitated oxalate.
- Resulting solution is heated, and CaO is added to form precipitate of calcium nitrate.
- Filtration and treated with H2SO4 to generate citric acid and precipitate of CaSo4.
- Filtration of dilute citric acid solution is decolorized and then it is evaporated to produce crystals of citric acid.
- Crystals recovered by centrifugation are, then dried and packaged.
USES
- Beverages: citric acid used in beverages industries to provide tartness and compliments of fruits, and also increase the effectiveness of antimicrobial preservatives.
- Jellies, jams and candy: It provide tartness to the products, involves in pH adjustment, and minimize sugar inversion.
- Frozen fruit: this acid lowers the pH to activate oxidative enzymes, and protects ascorbic acid by inactivating trace metals.
- Dairy products: it used as emulsifier in ice cream and processed cheese.
- Pharmaceutical: As effervescent in powders and tablets, also used as anticoagulant.
- Cosmetics and toiletries: In ph adjustment, buffering agent.
Reference and Sources
- 1% – https://issuu.com/walledashwah/docs/environmental_engineering_dictionar
- 1% – https://wikimili.com/en/Glucono_delta-lactone
- 1% – https://mafiadoc.com/production-of-organic-acids-from-agro-industrial-_59c1dbb11723ddbc5223b917.html
- 1% – http://europepmc.org/articles/PMC3769771/
- <1% – https://mafiadoc.com/world-journal-of-microbiology-and biotechnology_5c6a35f9097c4718438b45c5.html
- 1% – https://www.scielo.br/pdf/babt/v42n3/v42n3a01.pdf 2% – http://www.readbag.com/www2-hcmuaf-vn-data-quoctuan-biotechnology-for-agricultural-waste-utilization



2 thoughts on “Citric acid: Introduction, Fermentation, Recovery and Uses”
Nice and useful notes for industrial work
Thanks Arati keep supporting us