Amino acids: Structure, Optical activity and Classifications
Introduction
- Amino acids are group of organic compound containing carbon, hydrogen, oxygen, and nitrogen.
- A biomolecule is carbon based organic compound which is produced by living organisms.
- Amino acids are the building blocks or serve as monomers of protein.
- Proteins are the polymers of amino acids, with each amino acid residue joined by a specific type of covalent bond.
- As the name implies, it contains two functional groups- amino group and carboxyl groups.
- The amino group is basic while the carboxyl group is acidic in nature.
General structure
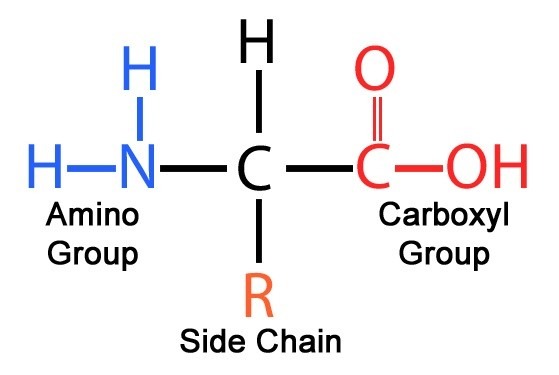
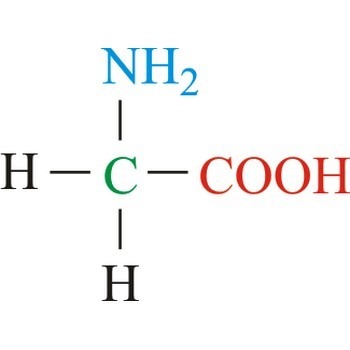
- The amino acid is termed as alpha amino acids if both the group, amino and carboxyl bonded to the same carbon atom.
- Various alpha amino acids differ from each other in their side chains or R groups, attached to their alpha carbon atom which vary in structure, shape, and electric charge and which influence the solubility of amino acids in water.
- The R group or side chains attached to the alpha carbon is different in each amino acid.
- the simplest amino acid is Glycine in which R group or side chain is hydrogen atom.
- There is exception of amino acid Proline, in which all alpha amino acids have the same structure.
Optical isomers of amino acids
- All amino acids except glycine, the alpha carbon atom is bonded to four different groups (a carboxyl group, an amino group, an R group and a hydrogen atom). The alpha carbon atom is thus a chiral center.
- Because of the tetrahedral arrangement of the bonding orbitals around the -carbon atom, the four different groups can occupy two unique spatial arrangements, and thus amino acids have two possible stereoisomers. Since they are non-super imposable mirror images of each other the two forms represent a class of stereoisomers called enantiomer
- all amino acids which have a chiral center are optically active that means they rotate the plane polarized light.
- An optically active compounds can rotate the plane polarized light either to the right (clockwise) or to the left (anticlockwise).
- Compounds that rotate the plane polarized light in clockwise direction are termed as dextrorotatory and designated by plus sign (+), while the compounds that rotate the plane polarized light in anti or counter clockwise are termed as levorotatory and designated by minus sign (-).
Standard and non-standard amino acids
Amino acids which are incorporated in the synthesis of protein are termed as standard amino acids, only 20 amino acids are known that are standard amino acids, while amino acids that are occurs in nature but not involved in the synthesis of protein are termed as non-standard protein.
Classifications of amino acids
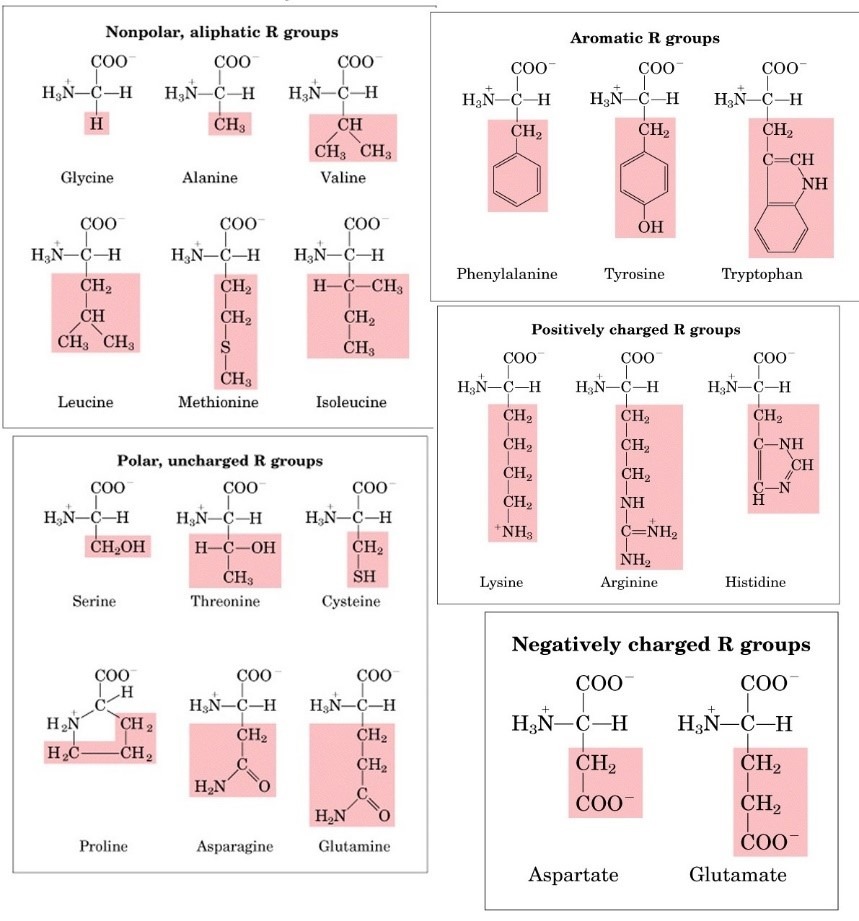
On the basis of structure:
- Amino acids with aliphatic side chains: These amino acids contain aliphatic groups e.g. glycine, leucine, valine etc.
- Amino acids containing hydroxyl groups: These amino acids contain hydroxyl groups e.g. serine, threonine and tyrosine.
- Sulfur containing amino acids: These are Sulphur containing amino acids e.g. cysteine, methionine.
- Acidic amino acids and their amide: Aspartic acids and glutamic acids are dicarboxylic monoacids while asparagine and glutamine are their respective amides derivatives.
- Basic amino acids: Lysine, arginine, and histidine are dibasic monocarboxylic acids.
- Aromatic amino acids: Phenylalanine, tyrosine, and tryptophan are aromatic amino acids.
- Imino acids: Proline is imino acids.
On the basis of polarity:
- Non-polar amino acids: Example- methionine, proline, alanine, leucine, valine, etc.
- Polar amino acids with no charge on R group: Example- threonine, glutamine, glycine, serine, threonine, glutamine.
- Polar amino acids with positive charge on R group: Example- histidine, and, lysine, arginine.
- Polar amino acids with negative charge on R group: Example- aspartic and glutamic acid.
On the basis of nutritional classifications:
- Essential amino acids: Amino acids which are not synthesized by body and needs to be supplied through diet. E.g. arginine, valine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan histidine, isoleucine.
- Non-essential amino acids: Amino acids that can be synthesized by the diet. E.g. glutamine, tyrosine, and proline, glycine, alanine, serine, cysteine, aspartate, asparagine, glutamate.
On the basis of metabolic fate:
- Glycogenic amino acids: Type of amino acid that can serve as precursor for the synthesis of glucose and glycogen. E.g. alanine, aspartate, methionine.
- Ketogenic amino acids: Amino acids that are involved in the synthesis of fat. E.g. leucine and lysine.
- Glycogenic and ketogenic amino acids: There are four amino acids that are involved in the synthesis of both fat and glucose. E.g. isoleucine, phenylalanine, alanine, tryptophan.
Also Read:
- Spectroscopy and its Types
- Amino acids: physical, chemical properties and peptide bond
- Proteins: Definition, Roles, Functions and Structure
- Plasmid: Properties, Types, Replication and Organization
- Classification of viruses on the basis of genome
- Enzymes: Introduction, Enzyme activity and work Mechanism
References and Sources
- https://en.wikipedia.org/wiki/Amino_acid
- https://www.scribd.com/presentation/359539330/Amino-acids-Peptides-and-Proteins-I-2013-ppt
- https://www.slideshare.net/shaguftaakmal/amino-acids-35650936
- https://www.slideshare.net/rohinisane/chemistry-of-amino-acids-with-their-clinical-applications
- https://biochemden.com/amino-acids-protein-fundamental-molecules/

It helps to recollect and remember thr properties and all the matter easily